
Now we will fill these electrons in different shells of the carbon atom.īefore we begin, we must understand the rules that are followed while filling the electrons in the shells. Therefore, the number of electrons in the carbon atom = 6 Just like protons, the number of electrons is also equal to the atom’s atomic number. For this, we would require calculating the number of electrons in this atom. Now, we will move forward to draw the shells of the carbon atom. Now that we know the number of protons and neutrons in the carbon atom, we will draw the nucleus of this atom which appears as follows: Hence, the number of neutrons in the carbon atom =12 ˗ 6 = 6 Now, we will put this in the formula mentioned above.

Rounding it up to the nearest whole number, we get 12. Using this formula, we will calculate the number of neutrons in the carbon atom. Now, we will calculate the number of neutrons in the carbon atom.įor an atom, the number of neutrons is calculated using the formula: Therefore, the number of protons in the carbon atom = 6 In the case of the carbon atom, atomic number = 6 The atomic number symbolizes the number of protons in an atom. For this, we will first have to calculate the number of protons and neutrons present in this atom. In the first step, we will draw the nucleus of the carbon atom. We will use this information to draw the Bohr model of the carbon atom.

The electrons are distributed in two shells, viz. In the case of the Carbon atom, there are 6 protons, 6 neutrons, and 6 electrons. These postulates given by Bohr, along with those given by Rutherford, came to be known as the Bohr˗Rutherford model, or simply, the Bohr model of the atom. These are known as valence electrons, and the shell is known as the valence shell. The electrons located in the outermost energy level participate in bond formation between different as well as similar atoms. Similarly, if an electron loses energy, it moves from higher to lower energy levels. As an electron absorbs energy, it moves from lower to higher energy levels. The electrons can also move from one orbit to another based on their energies. Therefore, the electrons housed in the shells located closest to the nucleus had minimum energy, while the electrons located in the outermost shell have maximum energy. He stated that the energies of these shells also increase from inside to outside. He named these shells K, L, M, N, etc., in alphabetical order and 1, 2, 3, 4, etc., in the numerical order increasing from inside to outside. He further stated that these shells have specific energies associated with them, which is why they are also known as energy levels. However, in 1916, Niel Bohr came out with the solution.īohr explained that the electrons do not move around the nucleus in random directions rather, they follow fixed orbits, also known as shells. Rutherford was unable to answer these queries, due to which this model was also about to get discarded. As this energy increases, it becomes impossible for the electrons to keep moving inside the radius of the atom. The question was that if the electrons were continuously in motion, they would slowly gain some acceleration and, thus, energy. The major argument against this model was about the stability of the atom. neutral neutrons, and positive protons.Īlthough this model explained most of the properties of an atom, it was accompanied by certain drawbacks.
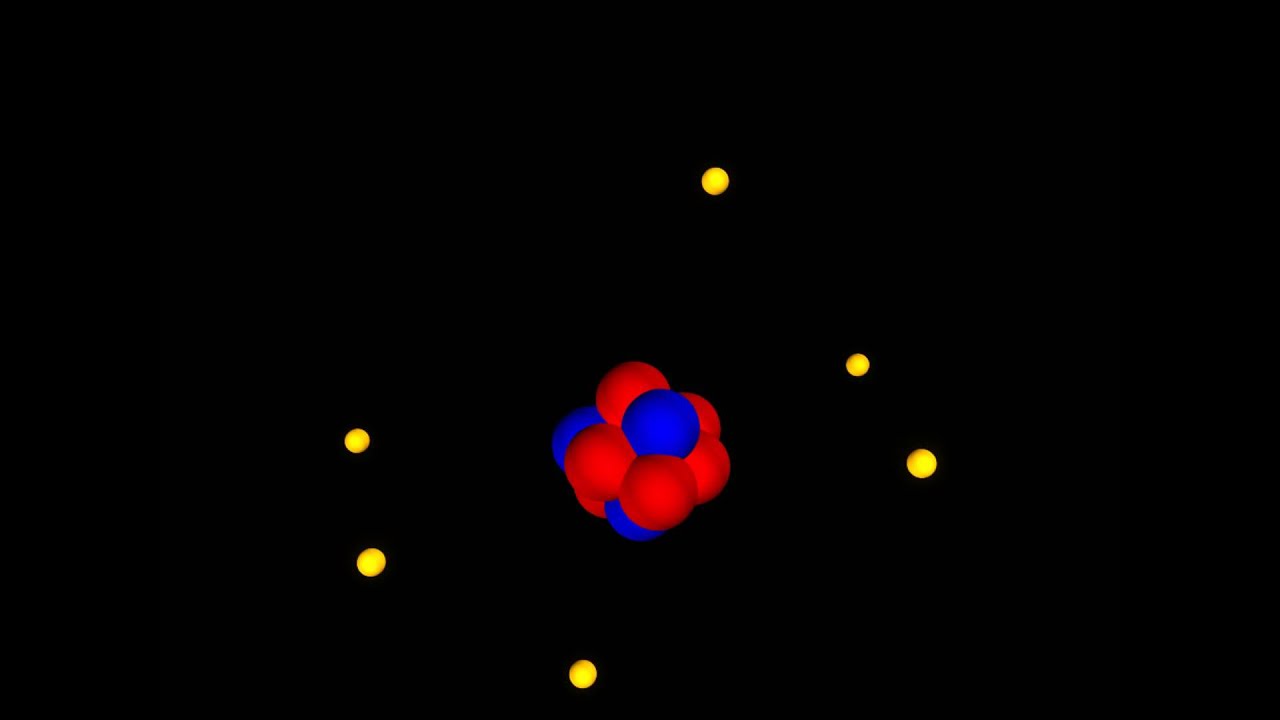
That is why this model was also known as the planetary model of atomic structure.Īlso, the nucleus of the atom was made up of two atomic species, viz. He explained that the atom is divided into a stationary positively charged nucleus and the negatively charged electrons that revolve around this nucleus, just like the planets around the sun in our solar system. After several failed attempts to explain the structure of the atom, Ernst Rutherford proposed his hypothesis in 1911.


 0 kommentar(er)
0 kommentar(er)
